We used self-reported data, which can introduce information bias, including misclassification, or effect bias exposure. Also, some participants might be more likely to report symptoms than others, and there is the potential for users to drop out of reporting in the app. Previous work has shown that data from our app is able to produce estimates of population-level disease prevalence that agree well with surveys with random, representative designs,18, 28 suggesting that behavioural issues are not substantially biasing our app population. However, given that strong reductions in COVID hospitalisations after vaccination were observed in nationwide studies in Scotland12 and England,11 we believe that collider bias is unlikely to underlie the reduction in infections seen in our data. Recipients of the ChAdOx1 nCoV-19 vaccine might differ from recipients of the BNT162b2 vaccine by age or dependency.
Although we adjusted for population differences across the BNT162b2, ChAdOx1 nCoV-19, and unvaccinated control groups, our estimates of infection rates after vaccination might not have fully adjusted for case-mix and therefore are preliminary. Furthermore, because the ChAdOx1 nCoV-19 vaccine started being rolled out in January, 2021, and the second dose is to be administered at 12 weeks, no app users had received two doses of ChAdOx1 nCoV-19 at the time of this report. The completeness of reporting was higher for systemic effects than for local effects, which might have introduced some bias . Some severe side-effects might have been missed if app users experiencing them were unable to use the app to report side-effects. However, we saw substantially lower rates of severe and mild side-effects than observed in phase 3 trials, making the missing of severe side-effects an unlikely explanation for the lower prevalence of side-effects seen in our data. Furthermore, we cannot rule out the presence of selection bias in who was tested after vaccination, as we know that health-care workers are tested more frequently than people in the general population, even if they are asymptomatic.
This is an observational study, with data captured during a specific timeframe, and our study design does not allow an inference of causality. Also, we evaluated only short-term adverse effects, and long-term surveillance in the general population will be required to investigate possible future effects. Finally, the systemic side-effects were collected from daily reports within 1 week from the injection date, so we cannot rule out that these effects might not be vaccine related.
We also had insufficient power to assess differential rates by ethnic group. The most common adverse reactions in clinical trial were typically mild fatigue and headache . Rare myocarditis and pericarditis, mostly in male adolescents and young adults . About 1 in 6 recipients reported adverse reactions after 2nd dose in clinical trial, especially fatigue or muscle pain . About 1 in 130,000 recipients required hospitalization for Guillain Barré syndrome, a neurological disorder in which the body's immune system damages nerve cells, causing muscle weakness and sometimes paralysis. Vaccine recipients did not report serious adverse reactions more often than placebo recipients.
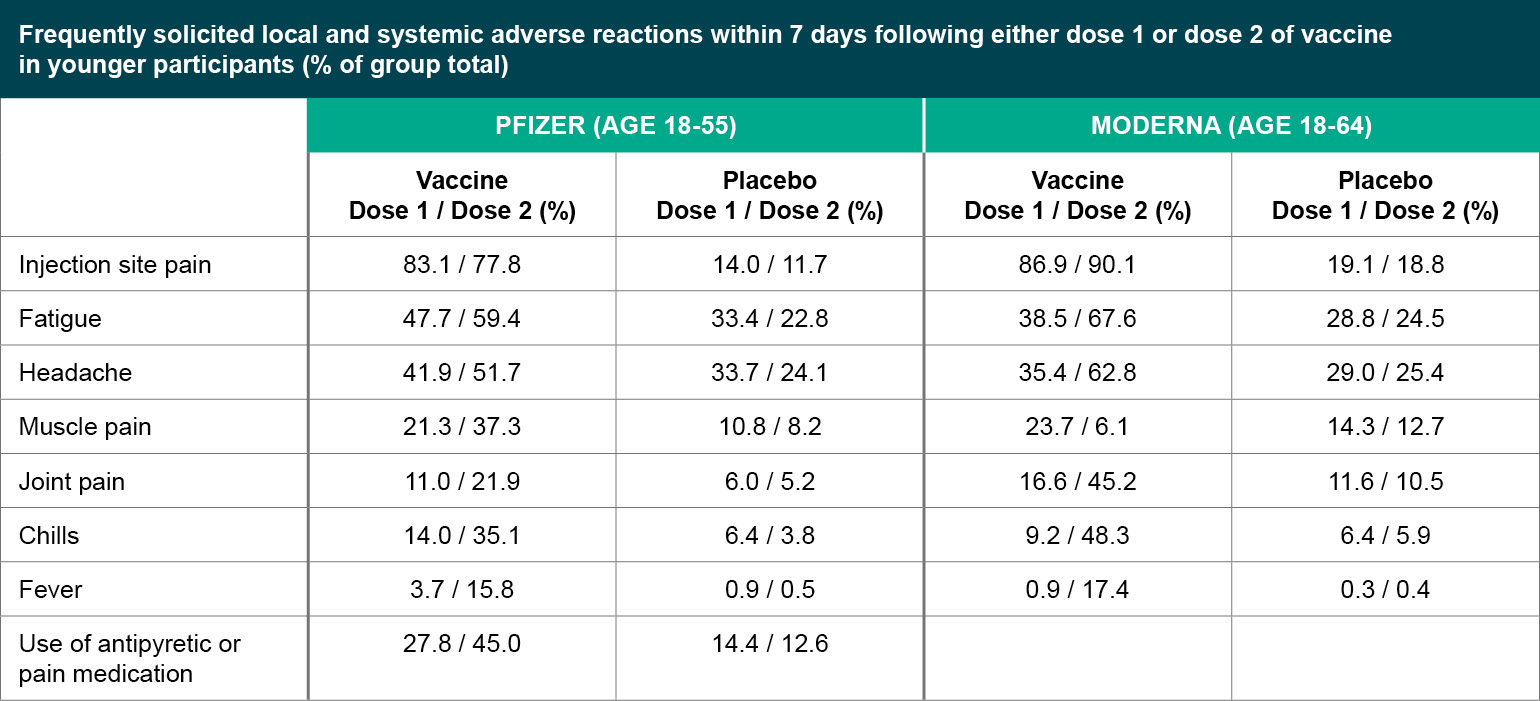
Mild to moderate reactions included soreness, headache, fatigue, muscle pain. Most common adverse reactions in clinical trials were flu-like illness, injection-site reactions, headache, and asthenia . Vaccine recipients did not report serious adverse events more frequently than placebo recipients .
The most common adverse reactions in the clinical trial were mild to moderate injection-site pain, headache and fatigue. "Serious and severe adverse events were low in number and balanced between vaccine and placebo groups." We observed an infection risk reduction at 21–44 days after vaccination in all vaccinated users compared with unvaccinated controls (RR was −69% [95% CI −72 to −66] for BNT162b2 and −60% [–68 to −49] for ChAdOx1 nCoV-19). Long-term surveillance for SARS-CoV-2 protection in individuals who have received delayed second doses of BNT162b2 compared with those receiving second doses according to initial guidelines will be required to determine whether these initial protection estimates persist. The frequency and severity of adverse events following vaccination with an mRNA COVID-19 vaccine in these populations were comparable to that of non-immunosuppressed individuals in these studies and what was reported in clinical trials. Safety data in these populations following vaccination with a viral vector vaccine is not available.
In studies with adults, the reactogenicity of a third dose of COVID-19 vaccine was similar to that of prior doses. The point estimates of vaccine efficacy at these two time points across a variety of age groups are similar to the overall estimate, including among study participants ≥65 years of age who comprised approximately 20% of the study population. Point estimates of vaccine efficacy at 14 days post-vaccination are comparable in study participants with and without one or more comorbidities. In contrast, the point estimate of efficacy in participants with comorbidities is somewhat lower at 28 days post-vaccination. Efficacy for Janssen vaccine was based on clinical trials that were conducted in countries with widely circulating VOCs , which may have impacted its overall efficacy. This is in contrast to the clinical trials for other authorized COVID-19 vaccines.
It is unclear to what extent pre-existing immunity to any adenovirus-based vaccine vector exists in the Canadian population and what impact that could have on adenovirus based vaccine safety and efficacy. It is also unclear as to what extent immunization with adenovirus-based vaccines elicits anti-vector immune responses and what impact that could have on homologous or heterologous booster doses with adenovirus-based vaccines. Evidence for a viral vector vaccine based on human adenovirus 5 indicated that vaccine recipients with high pre-existing immunity to the adenovirus vector had lower anti-SARS-CoV-2 immune responsesFootnote 136. The AstraZeneca COVID-19 vaccine uses a modified chimpanzee adenovirus vector . AstraZeneca found no correlation between anti-ChAd neutralizing antibody responses and anti-SARS-CoV-2 immune responses. It also found that neutralizing antibody levels were not boosted after the second dose.
However, neutralization is not the only anti-vector immune response that could impact vaccine-induced immunity. It remains unclear if immune responses to the ChAd vector will impact the efficacy or effectiveness of this vaccine. Pain at the injection site is very common after administration of the currently authorized, COVID-19 vaccine. Localized axillary swelling and tenderness was a solicited adverse event in the Moderna COVID-19 clinical trial and was very common after administration with that vaccine. Local adverse events are usually mild or moderate and resolve within a few days of vaccination.
For the authorized mRNA COVID-19 vaccines, pain at the injection site was slightly more frequent in younger authorized age groups including adolescents years of age (Pfizer-BioNTech COVID-19 vaccine) and years of age (Moderna COVID-19 vaccine) compared to older adults. For AstraZeneca COVID-19 vaccine, local reactions were milder and reported less frequently after the second vaccine dose in all age groups. Similar frequencies of local reactions were reported across age groups after administration of the Janssen vaccine. Evidence for a COVID-19 viral vector vaccine based on human adenovirus 5 indicated that vaccine recipients with high pre-existing immunity to the adenovirus vector had lower anti-SARS-CoV-2 immune responsesFootnote 136. Janssen found no correlation between anti-Ad26 neutralizing antibody responses and anti-SARS-CoV-2 immune responses. It remains unclear if immune responses to the Ad26 vector will impact the efficacy or effectiveness of this vaccine.
Rare cases of serious blood clots, including cerebral venous sinus thrombosis, associated with thrombocytopenia have been reported in Canada and globally following post-licensure use of AstraZeneca COVID-19 vaccine. Cases have usually occurred between 4 and 28 days after receipt of vaccine. This adverse event is being referred to as Vaccine-Induced Immune Thrombotic Thrombocytopenia .
The mechanism of action is similar to heparin-induced thrombocytopenia . The exact mechanism by which the viral vector COVID-19 vaccines may trigger VITT is still under investigation. At this time, no other predisposing factors have consistently been identified in patients who develop VITT. The rate of this adverse event is still to be confirmed, but had been most commonly estimated to be between 1 in 26,000 persons and 100,000 persons vaccinated with a first dose of AstraZeneca COVID-19 vaccine although this continues to evolve and may increase. Based on available evidence as of June 1, 2021, PHAC has estimated the rate of VITT in Canada to be 1 in 73,000 doses administered. However, as investigations continue, this rate could be as high as 1 in 50,000 persons vaccinated with the AstraZeneca/COVISHIELD COVID-19 vaccine.
The frequency of TTS following a second dose of AstraZeneca vaccine is currently reported to be approximately 1 per 520,000 individuals vaccinated with a second dose, based on vaccine safety surveillance data from the United Kingdom but this continues to evolve Footnote 2. Additional information is currently being gathered to characterize the rate of VITT more accurately. Based on available information, the case fatality of VITT typically ranges between 20 and 50%. Case fatality may vary with increased awareness of the adverse event and appropriate early treatment. The onset of new autoimmune disease or disease exacerbation following vaccination with mRNA COVID-19 vaccines was rare or comparable to the background incidence of these events in the general population.
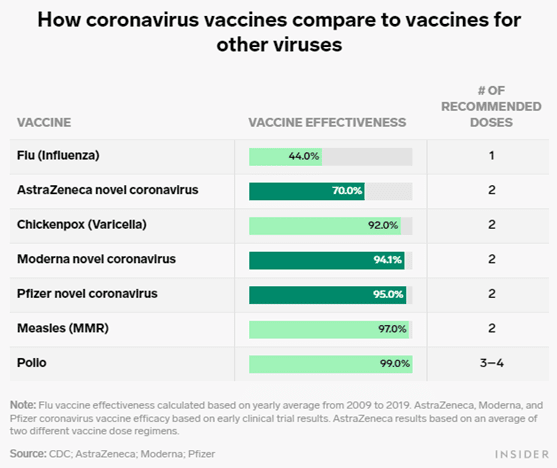
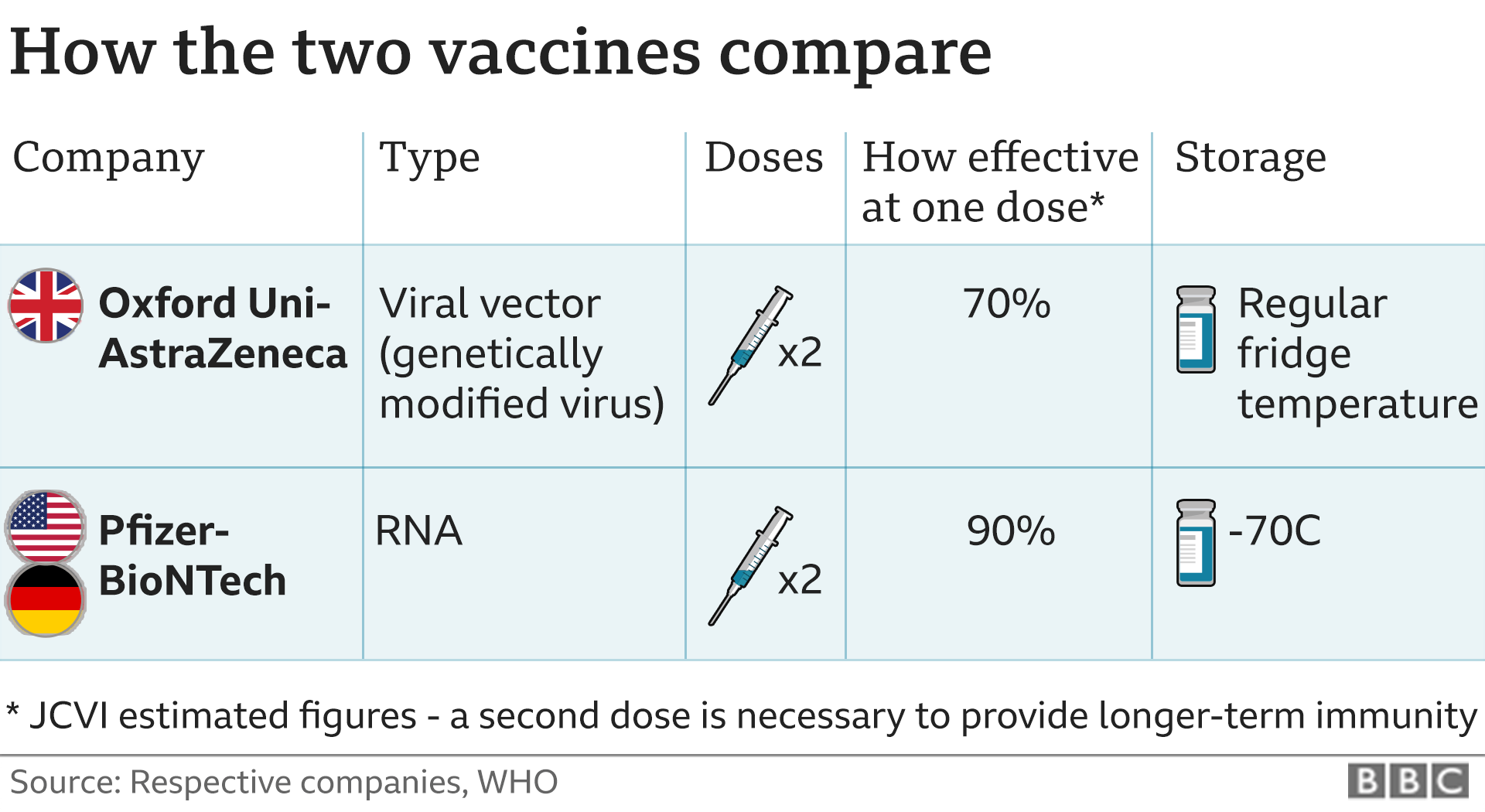
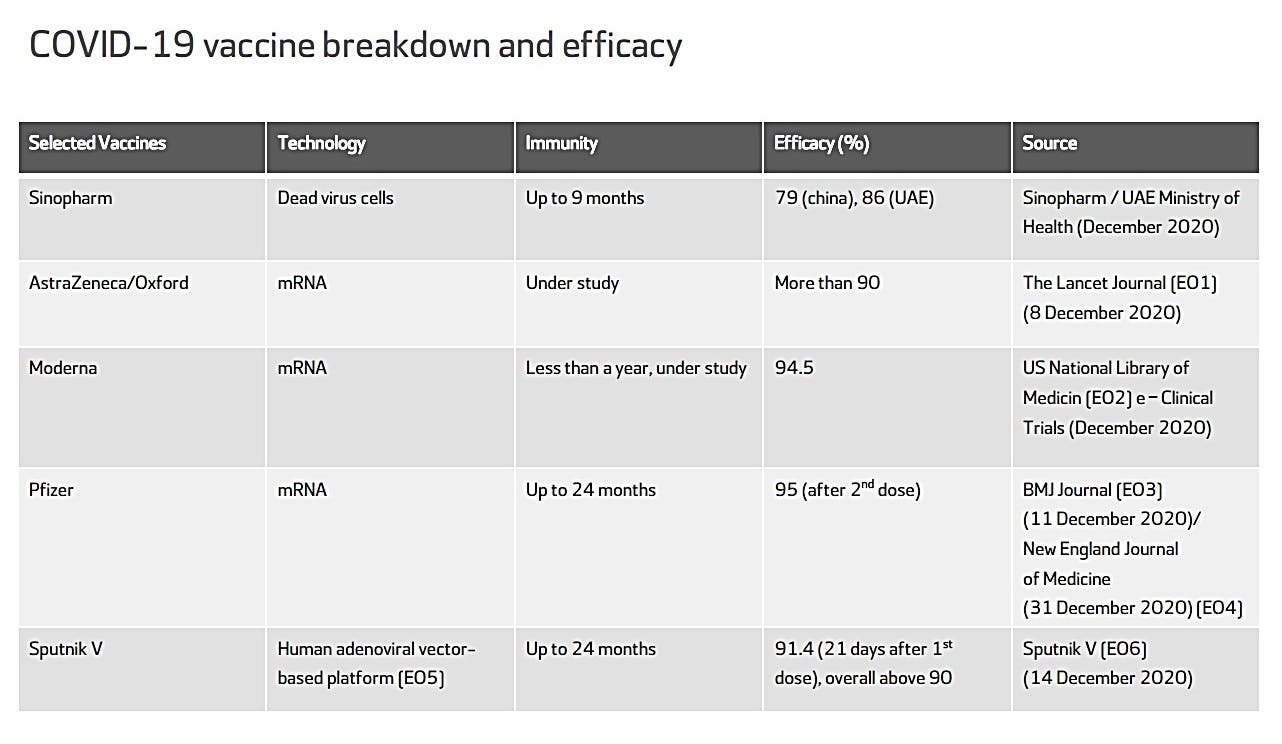
Combined evidence from clinical trial and observational study data available to date have shown that the AstraZeneca COVID-19 vaccine offers protection against symptomatic COVID-19 disease and hospitalization in adults ≥18 years of age after receiving at least one dose. Clinical trial data available to date have shown that the AstraZeneca COVID-19 vaccine has demonstrated moderate efficacy against symptomatic, confirmed COVID-19 of approximately 62% in those years of age. Efficacy of a two-dose series increased to approximately 82% when the interval between doses was 12 weeks or more. In adults 65 years of age and over, observational data from the UK of vaccine effectiveness after one dose have shown a reduction in the risk of symptomatic disease and hospitalization. Evidence on the safety of vaccine booster doses is available from observationalFootnote 25 and clinical studiesFootnote 26 Footnote 27 Footnote 28.
Occurrence of solicited and unsolicited systemic adverse events in individuals with prior SARS-CoV-2 infection was slightly higher compared to the SARS-CoV-2 naïve population, primarily in younger adults. However, there was no observed increase in the frequency of more severe adverse events in this population. Two observational studies included less than 100 patients with persistent symptoms from prior COVID-19 infections . In this subgroup, receipt of COVID-19 vaccination with either an mRNA or viral vector vaccine was not associated with a worsening of long COVID symptoms or increased reactogenicity following immunization. Fatigue, headache, muscle pain, chills, and joint pain are all either common or very common after the administration of the currently authorized, COVID-19 vaccines.
Fever was very common after administration of the second dose of the mRNA COVID-19 vaccines and common after any dose of viral vector COVID-19 vaccines. More than a quarter of vaccine recipients experienced headache, and/or fatigue after any dose. For the mRNA COVID-19 vaccines, systemic adverse events are usually mild or moderate intensity and resolve within a few days of vaccination.
Systemic reactions are more frequent after the second vaccine dose and in younger authorized age groups including adolescents years of age (Pfizer-BioNTech COVID-19 vaccine). For AstraZeneca COVID-19 vaccine, systemic reactions are milder and reported less frequently after the second vaccine as compared with the first in all age groups. The frequencies of systemic reactions that were reported after administration of the Janssen vaccine were similar across age groups. Exploratory analyses for the AstraZeneca viral vector vaccine has not demonstrated efficacy against confirmed SARS-CoV-2 asymptomatic infection, however the number of asymptomatic infections was small. The clinical trial of the Janssen COVID-19 vaccine found the vaccine to have moderate protection against asymptomatic and undetected COVID-19 infection. Pfizer-BioNTech and Moderna have reported the racial/ethnic composition of the participants in the late-stage clinical trials for their COVID-19 vaccines.
Pfizer-BioNTech and Moderna provided demographic data for participants in their late-stage clinical trials, including racial/ethnic composition, as part of their emergency use authorization applications to the FDA. These data show, that, overall, people of color are underrepresented in these trials relative to their share of the total U.S. population , with the largest disparity among the Black population. While the trials have not included the overrepresentation of people of color that some had suggested, as noted above, these trials have achieved greater diversity than many previous trials for other drugs. In both COVID-19 trials, the demographics of the placebo and vaccine groups are similar, as are the characteristics between all participants and the safety populations . In addition, similar vaccine efficacy results were observed across racial and ethnic groups in both the Pfizer and Moderna trials.
Safety data in this population following vaccination with a viral vector vaccine is not available. The impact of immunocompromise on seroconversion after vaccination varies according to specific conditions and/or immunosuppressive therapy. Some studies have shown that immunogenicity is substantially decreased in some immunocompromised adults when compared to healthy vaccine recipients. This notably included individuals with malignancy , solid organ transplant recipients, and those with primary immune deficiency.
Given the lack of a defined immunological correlate of protection against SARS-CoV-2 infection, the clinical significance of this difference in seroconversion and its impact on vaccine effectiveness is not known. Evidence on the safety of COVID-19 vaccination of individuals with prior SARS-CoV-2 infection is available from observationalFootnote 25Footnote 58Footnote 59 and clinical studiesFootnote 26Footnote 27Footnote 28. The occurrence of solicited and unsolicited systemic adverse events after the first or second dose in individuals with prior SARS-CoV-2 infection was slightly higher compared to the SARS-CoV-2 naïve population. Clinical trial data show that efficacy increased as the interval between doses increased.
At present, there are insufficient clinical trial data in adults ≥65 years of age to assess vaccine is efficacy in this age group. The vaccine is similarly efficacious in adults ≥18 years of age with and without pre-defined comorbidities . Diversity within clinical trials for a COVID-19 vaccine helps ensure safety and effectiveness across populations and may increase confidence in getting the vaccine among people of color. Historically, people of color have been underrepresented in clinical trials.
There have been recent efforts to increase racial diversity within clinical trials and specific efforts to increase diversity within the trials associated with development of COVID-19 vaccines. The key parameter in our model is the relative efficacy provided by the first dose of vaccine compared to the level of protection offered by two doses. Here, we have focused on COVID-19 mortality using the relative risk of infection followed by death for each of the nine JCVI priority groups, and hence we are most interested in efficacy against death. For other variants of concern the uncertainty in vaccine efficacy is even greater. In a subgroup analysis in study participants who received the SD/SD vaccine regimen, vaccine efficacy against confirmed COVID-19 cases occurring at ≥15 days after dose 2 was estimated by dosing interval and age group. These ad-hoc subgroup analyses were performed in participants years of age from the COV002 clinical trial and in all study participants who received the SD/SD regimen , dichotomized into groups years and ≥65 years of age.
The following populations were either excluded from or were represented by small numbers of participants in the original pivotal clinical trials. NACI has updated recommendations for these populations as real-world evidence has become available. The recommendations above on the use of mRNA COVID-19 vaccines (Recommendation #1) and the use of viral vector COVID-19 vaccines (Recommendation #2), also apply to those who are immunosuppressed, have autoimmune conditions, are pregnant or are breastfeeding.
The clinical trial data demonstrates that the authorized mRNA COVID-19 vaccines are efficacious over the short-term in individuals with or without evidence of prior SARS-CoV-2 infection. The efficacy of the Janssen COVID-19 vaccine in those with evidence of prior infection is inconclusive at this time due to small sample size, and this outcome has not been assessed for AstraZeneca COVID-19 vaccine. They also cited data from a Nature study and a report from the Israel Ministry of Health suggesting that the vaccine's efficacy for both preventing infection and symptomatic disease decreased six months after vaccination, but still prevented serious illness and death.
In conclusion, short-term adverse effects of both vaccines are moderate in frequency, mild in severity, and short-lived. Adverse effects are more frequently reported in younger individuals, women, and among those who previously had COVID-19. Our data could be used to inform people on the likelihood of side-effects on the basis of their age and sex and the type of vaccine being administered. Furthermore, our data support results from randomised controlled trials in a large community-based scenario showing evidence of reduction in infection after 12 days and substantial protection after 3 weeks. No study investigated the prevalence of adverse effects of the vaccines and all studies reported both vaccines to be highly effective.
Safety evidence is based on interim analyses of 23,745 participants of which 12,021 received at least one dose of the AZ COVID-19 vaccine and 11,724 received a control. Solicited adverse events occurring within 7 days after any dose were assessed among 2648 vaccine recipients who received at least one dose and 2497 control recipients. Approximately one third of study participants received their second vaccine dose within 6 weeks of receiving Dose 1. The majority (~90%) of study participants in the safety cohort were less than 65 years of age. The median duration of follow-up was 105 days post-Dose 1 and 62 days post-Dose 2.
The highest efficacy with the authorized regimen of AstraZeneca COVID-19 vaccine was seen in clinical trial groups that had a longer interval between doses. Clinical trials suggest that vaccine efficacy increases with extended intervals between the first and second dose of vaccine, with a maximum reduction in risk of symptomatic disease and hospitalization observed at 12 weeks or more following the priming dose. There are currently no data on the efficacy or effectiveness of an additional dose of a COVID-19 vaccine following a 1- or 2-dose primary series in individuals with immunocompromising conditions.
Emerging evidence indicates that humoral immune responses increase after a third dose of mRNA COVID-19 vaccines is administered to adults with immunocompromising conditions, although the degree of increase varies according to the type of immunocompromising condition or treatment. In some studies, although the increase in proportion of those who seroconverted was small, median antibody titers increased after the third dose compared to after the second dose. There was a significant amount of heterogeneity between studies due to differences in the populations that were studied. Given the limited size of the studies available to date and the lack of a defined immunological correlate of protection, there are limitations to interpreting the significance of these results. For all vaccines, some solicited adverse events were reported to be very common (defined as 10% or more) among vaccine recipients. However, they are mild or moderate and transient, resolving within a few days.
These include pain at the injection site, fatigue, headache, muscle pain, chills, joint pain, and fever. In clinical trials of mRNA vaccines, some adverse events, including fever, are more frequent after the second dose; this was not the case with the AstraZeneca COVID-19 vaccine. Rare cases of serious blood clots, including cerebral venous sinus thrombosis, associated with thrombocytopenia have been recently reported in the United States following post-licensure use of Janssen COVID-19 vaccine. This adverse event is being referred to as Vaccine-Induced Immune Thrombotic Thrombocytopenia and has been associated with both the AstraZeneca and Janssen COVID-19 viral vector vaccines. As of May 24, 2021, 32 cases of TTS out of about 10.2 million doses of Janssen administered in the United States have been confirmed.
Most of the cases to date have occurred in females between the ages of 18 and 49 years; however investigations are ongoing and additional cases may be identified with increased awareness and current emphasis on the clinical recognition of this event. Reports indicated symptom onset between 6 and 15 days after vaccination. In participants with and without comorbidities, efficacy was assessed against confirmed symptomatic moderate to severe/critical and against severe/critical COVID-19 infection with onset ≥14 days and ≥28 days post-vaccination . An exploratory analysis examined the potential effect of the interval between the administration of the first and second vaccine doses on vaccine efficacy in study participants receiving the SD/SD vaccine regimen. Table 10 summarizes the estimates of vaccine efficacy against confirmed COVID-19 cases occurring at ≥15 days after dose 2 by dosing interval.





















No comments:
Post a Comment
Note: Only a member of this blog may post a comment.